Change Occurs Only if the Surroundings Continuously Supply the System With an Input of Energy
Thanks to physics, we know that p hase changes occur when materials change state, going from liquid to solid (as when water freezes), solid to liquid (as when rocks melt into lava), liquid to gas (as when you boil water for tea), and so on. When the material in question changes to a new state — liquid, solid, or gas (you can also factor in a fourth state: plasma, a superheated gas-like state) — some heat goes into or comes out of the process without changing the temperature.
You can even have solids that turn directly into gas. As dry ice (frozen carbon dioxide gas) gets warmer, it turns into carbon dioxide gas. This process is called sublimation.
Imagine you're calmly drinking your lemonade at an outdoor garden party. You grab some ice to cool your lemonade, and the mixture in your glass is now half ice, half lemonade (which you can assume has the same specific heat as water), with a temperature of exactly 0 degrees Celsius.
As you hold the glass and watch the action, the ice begins to melt — but the contents of the glass don't change temperature. Why? The heat (thermal energy) going into the glass from the outside air is melting the ice, not warming the mixture up. So does this make the equation for heat energy
![]()
useless? Not at all — it just means that the equation doesn't apply for a phase change.
If you graph the heat added to a system versus the system's temperature, the graph usually slopes upward; adding heat increases temperature. However, the graph levels out during phase changes, because on a molecular level, making a substance change state requires energy. After all the material has changed state, the temperature can rise again.
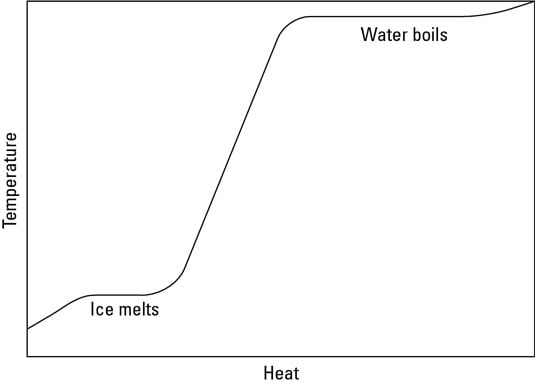
Phase changes of water.
Imagine that someone has taken a bag of ice and thoughtlessly put it on the stove. Before it hit the stove, the ice was at a temperature below freezing (–5 degrees Celsius), but being on the stove is about to change that. You can see the change taking place in graph form in the figure. (The specific heat of ice is around 2.1 x 103 J/kg-C)
![]()
that the temperature of the ice will increase linearly as you add more heat to it, as you see in the graph.
However, when the ice reaches 0 degrees Celsius, the ice is getting too warm to hold its solid state, and it begins to melt, undergoing a phase change. When you melt ice, breaking up the crystalline ice structure requires energy, and the energy needed to melt the ice is supplied as heat. That's why the graph in the figure levels off in the middle — the ice is melting. You need heat to make the ice change phase to water, so even though the stove adds heat, the temperature of the ice doesn't change as it melts.
As you watch the bag of ice on the stove, however, you note that all the ice eventually melts into water. Because the stove is still adding heat, the temperature begins to rise, which you see in the figure. The stove adds more and more heat to the water, and in time, the water starts to bubble. "Aha," you think. "Another phase change." And you're right: The water is boiling and becoming steam. The bag holding the ice seems pretty resilient, and it expands while the water turns to steam.
You measure the temperature of the water. Fascinating — although the water boils, turning into steam, the temperature doesn't change. Once again, you need to add heat to incite a phase change — this time from water to steam. You can see in the figure that as you add heat, the water boils, but the temperature of that water doesn't change.
What's going to happen next, as the bag swells to an enormous volume? You never get to find out, because the bag finally explodes.
Source: https://www.dummies.com/article/academics-the-arts/science/physics/why-temperature-remains-constant-during-a-phase-change-174234/
0 Response to "Change Occurs Only if the Surroundings Continuously Supply the System With an Input of Energy"
Post a Comment